CAR-T CELLS THERAPY
A.Tsyb Medical Radiological Research Center (MRRC) contact center:
+7 (800) 250 – 87 – 00
P. Hertsen Moscow Oncology Research Institute (MORI) contact center:
+7 (495) 150 – 11 – 22
Biomedical cell product “T-cell with chimeric antigen receptor (CAR) to the CD19 antigen”. Produced by FSBI NMRRC of Ministry of Health of Russia on individual prescription.
CAR-T is a cell therapy using genetically modified cells of the patient’s own immune system. In this treatment option, by means of nanotechnology T-lymphocites (of the human body’s cells-protectors) are delivered a “weapon” that is specifically targeted against a certain disease of a patient.
CAR-T helps achieve a response to treatment even in cases of resistibility to chemoradiotherapy, targeted medications, as well as in disease recurrence.
The use of this technology was first introduced in the treatment of hemato-oncological diseases (blood cancers and lymphoid tissue). Presently, treatment methods with CAR-T cells are being developed for both cancer diseases and non-tumor diseases (autoimmune pathology, AIDS).
What is CAR-T cell therapy?
This method is designated for cell therapy B-cell hemato-oncological diseases: progressing or relapsing resistant forms of non-Hodgkin’s lymphomas and acute lymphoblastic leukemia.
T-lymphocytes composing the drug formula carry on their surfaces chimeric antigen receptor for the differentiation marker CD19 (anti-CD19-CAR). Thanks to it lymphocytes are capable of cytotoxic reaction against tumor cells and fast suppression of tumor niduses.
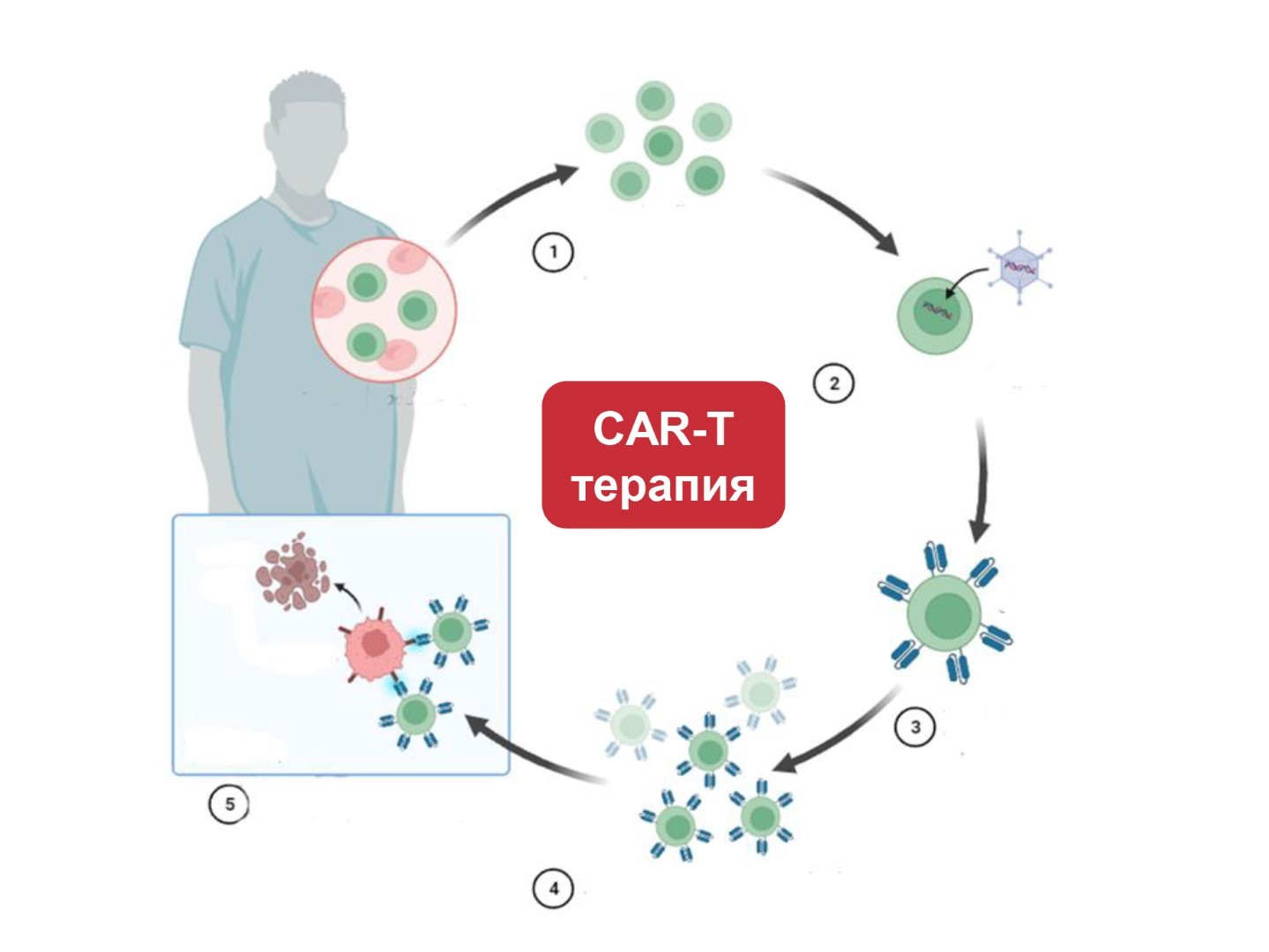
CAR-T cell therapy phases
CAR-T therapy consists of several phases:
- Evaluation of patient’s conditions and tumor characteristics;
- collection of patient’s lymphocytes;
- modification (introduction) of patient’s T- lymphocytes by means of genetically manipulated receptor directed specifically against patient’s tumor;
- multiplying obtained T-cells in the special incubator;
- return of modified CAR-T-lymphocytes to the patient.
Treatment results
Treatment results are often overwhelming, because even in multiple resistance (non-effectiveness of previous treatment) or with repeated relapses obtainment of results with significant decrease of tumor lesion is possible and capabilities of reinforcement of the achieved effect.
Currently at A.Tsyb MRRC – branch of FSBI NMRRC of Ministry of Health of the Russian Federation obtained the first and so far the only operating license to use this technology. The practical use of this method in clinical practice for treatment of CD19-positive lymphomas and leucosis has started. It is planned to expand the range of diseases and the list of cellular products for their treatment.
Selection of patients for this type of treatment is carried out exclusively by hematologists and oncologists after full-range examination.
Referral of patients for consultation may be carried out in-person or in the mode of telecommunication consultation with the institution where the patient is currently being treated.